January 2, 2023
#2719: Hydrogen Isotopes explain
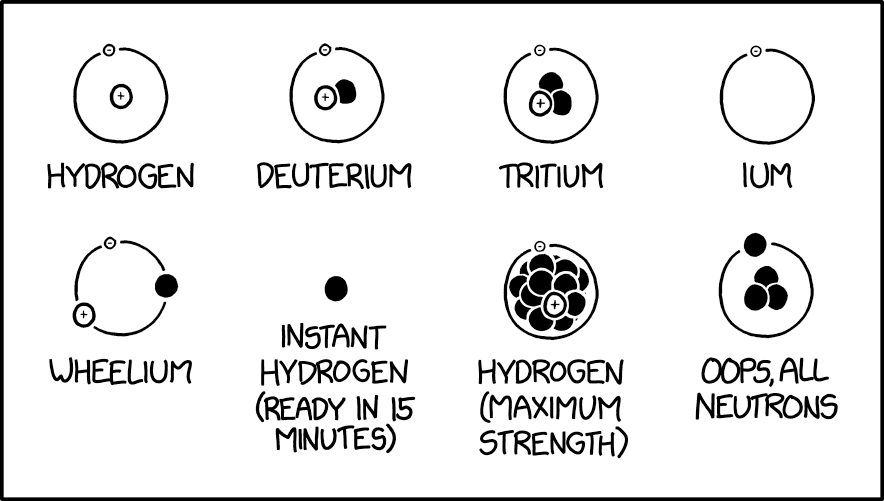
[Eight drawings of different versions of hydrogen atoms are shown. They are arranged in two rows of four. The depictions use the planetary model version with for instance a negative electron (with a “-” written inside a small circle) orbiting a positive proton (with a “+” written inside a larger circle) and a black neutron depicted as a circle of the same size as the neutron, as in the second atom - Deuterium. Each has a label underneath. Here, they are listed in reading order:]
[An electron orbiting a proton:]
Hydrogen
[An electron orbiting a proton connected with a neutron:]
Deuterium
[An electron orbiting a proton connected with two neutrons, so that they form a triangle:]
Tritium
[An electron orbiting nothing:]
Ium
[An electron, a proton and a neutron placed equidistant from each other on the same circular orbit around nothing:]
Wheelium
[A single neutron:]
Instant Hydrogen (ready in 15 minutes)
[An electron orbiting a proton connected with many neutrons, 13 visible with six touching the proton which are in front. Four more are close to those six and mostly shown and then three are only just visible behind the others. Looking closely there are also two smaller dots near the edge indicating at least two more, for 15 that can be seen. And several more would be behind the visible neutrons if this forms a spherical shape. The electron’s orbit just barely goes around the outer neutrons:]
Hydrogen (maximum strength)
[Four neutrons, arranged like the particles in Tritium but with a neutron orbiting a triangle of neutrons.]
Oops, All Neutrons