November 3, 2014
#1442: Chemistry explain
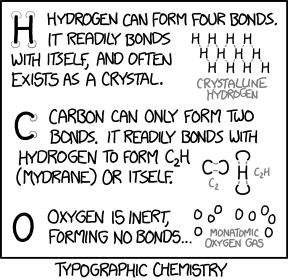
[A large capital letter “H”, with faint gray circles drawn on the ends of each of the four legs.]
Hydrogen can form four bonds. It readily bonds with itself, and often exists as a crystal.
[A lattice of several H’s, all “bonded” together at the ends of their legs in a crisscross, meshlike pattern, labeled:]
Crystalline hydrogen
[A large capital letter “C”, with faint gray circles drawn on both ends of the arc.]
Carbon can only form two bonds. It readily bonds with hydrogen to form C2H (mydrane) or itself.
[Image of a C and an inverted C, linked at their endpoints, labeled:]
C2
[Image of two C’s linked with an H between them, labeled:]
C2H
[A large capital letter “O”.]
Oxygen is inert, forming no bonds…
[Image of several lone O’s, none connected to anything, labeled:]
Monatomic oxygen gas.
[Caption at bottom:]
Typographic chemistry